What is Radioactive Dating? 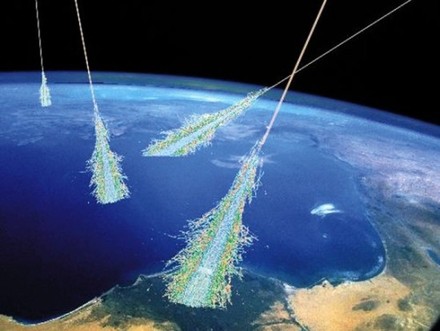 Cosmic rays bombard Earth’s atmosphere, creating the unstable isotope carbon-14. This isotope lets scientists learn the ages of once-living specimens from long ago. Image via The Cosmic Story of Carbon-14 by Ethan Siegel, via Simon Swordy (U. Chicago), via NASA Radiocarbon dating uses carbon isotopes. Radiocarbon dating relies on the carbon isotopes carbon-14 and carbon-12. Scientists are looking for the ratio of those two isotopes in a sample. Most carbon on Earth exists as the very stable isotope carbon-12, with a very small amount as carbon-13. Carbon-14 is an unstable isotope of carbon that will eventually decay at a known rate to become carbon-12. Cosmic rays – high energy particles from beyond the solar system – bombard Earth’s upper atmosphere continually, in the process creating the unstable carbon-14. Carbon-14 is considered a radioactive isotope of carbon. Because it’s unstable, carbon-14 will eventually decay back to carbon-12 isotopes. Because the cosmic ray bombardment is fairly constant, there’s a near-constant level of carbon-14 to carbon-12 ratio in Earth’s atmosphere. Organisms at the base of the food chain that photosynthesize – for example, plants and algae – use the carbon in Earth’s atmosphere. They have the same ratio of carbon-14 to carbon-12 as the atmosphere, and this same ratio is then carried up the food chain all the way to apex predators, like sharks. But when gas exchange is stopped, be it in a particular part of the body like in deposits on bones and teeth, or when the entire organism dies, the ratio of carbon-14 to carbon-12 begins to decrease. The unstable carbon-14 gradually decays to carbon-12 at a steady rate. And that’s the key to radiocarbon dating. Scientists measure the ratio of carbon isotopes to be able to estimate how far back in time a biological sample was active or alive. Adapted from EarthSky in FAQs | Earth | Human World on Jan 17, 2014
0 Comments
Leave a Reply. |
AuthorWrite something about yourself. No need to be fancy, just an overview. Archives
August 2014
Categories |
| SCIENCE Level 1 |
|
 RSS Feed
RSS Feed
